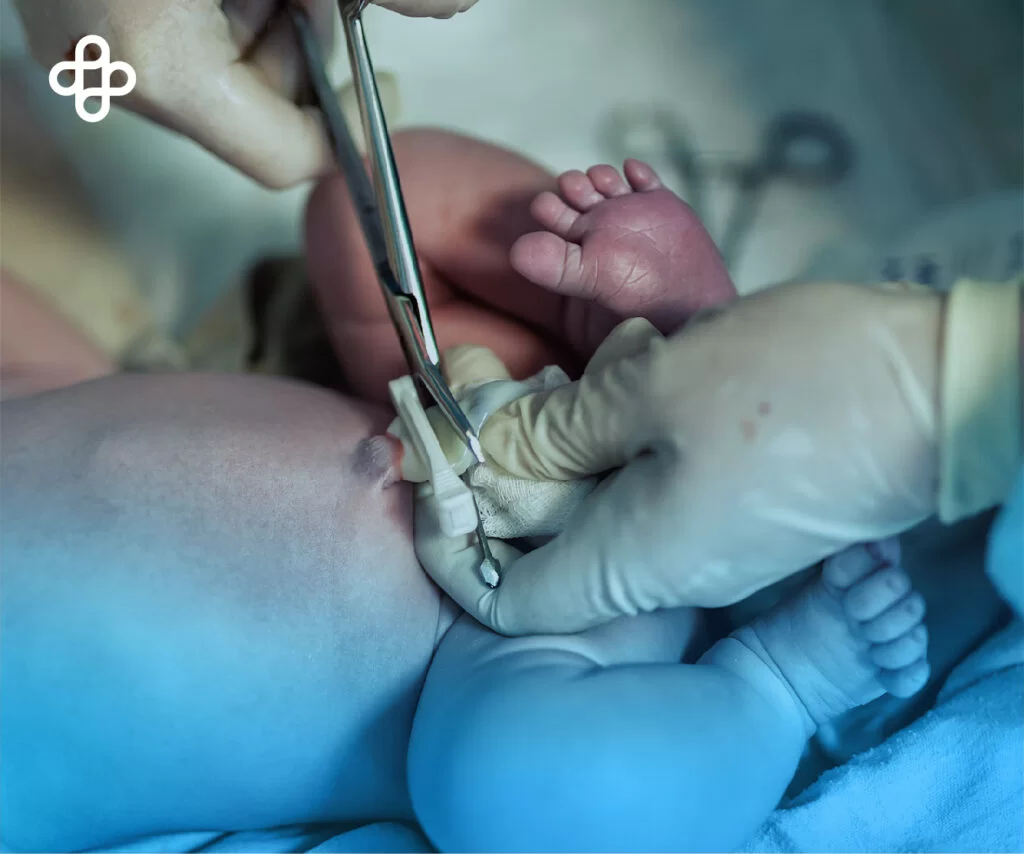
Umbilical cord blood may offer a therapeutic alternative in treating cerebral palsy. A study conducted by the University of Bern in Switzerland has shown encouraging results.
Cerebral palsy occurs in two out of every thousand births, leading to lifelong neurological deficits with no treatment available other than general support measures. It most commonly arises due to perinatal hypoxemia, prematurity, and intrauterine growth restriction.
An emerging therapeutic alternative is the use of stem cells from umbilical cord blood.
The Department of Obstetrics at the University of Bern, Switzerland, performed a meta-analysis that made this evident. The analysis collected published studies demonstrating the utility of umbilical cord blood stem cells (UCB) in treating cerebral palsy.
To be eligible for this therapy, it is necessary to have preserved umbilical cord blood at the time of birth. However, until now, it was unclear whether it was possible to collect cord blood cells in at-risk cases. Collection in high-risk patients is challenging.
One reason is that in obstetric emergencies, planning for collection is not feasible. Additionally, in premature infants, delayed cord clamping and anatomical conditions may reduce availability. Thus far, most existing trials have used donor cells.
A recent study (2021) concludes that the collection of umbilical cord blood in neonates at risk of brain damage is feasible.

Study Design
The study aimed to assess the feasibility of collecting umbilical cord blood in newborns at risk of brain damage.
Neonates with any of the following high-risk criteria were included: symptomatic perinatal hypoxemia, extreme prematurity (23-30 weeks or estimated weight less than 1,500 grams), intrauterine growth restriction estimated below the third percentile for gestational age weight, or monochorionic twins with twin-twin transfusion syndrome.
Stem cells hold the potential to treat a wide range of diseases, including cerebral palsy.
International Society for Stem Cell Research
They aimed to collect and preserve it according to the highest standards. Units were cryopreserved as whole blood in the vapor phase of liquid nitrogen at temperatures between -150ºC and -185ºC.
Furthermore, to ensure dedication and compliance with the project, all midwives and obstetricians received training on blood collection following rigorous procedures.
Results
In this study, attempts were made to collect umbilical cord blood in 177 cases, and it was successfully done in 141 of them (80%).
In 105 cases, the quantity and quality of the cord blood were adequate for therapeutic use.
Nearly all instances of insufficient collection occurred in preterm infants (less than 30 weeks) or those weighing less than 1,500 grams. Of the 177 at-risk newborns, 10% showed signs of brain damage.
Thereafter, be sure to visit our article What are stem cells?